Which of These Compounds Is a Strong Electrolyte
A solution which does not conduct electricity is known as a non-electrolyte. On the other hand ionic compounds are a part of the bigger family of the polar compounds.

What Are Some Examples Of A Strong Electrolyte Quora
To a certain extent the way we name a compound depends on the type of electrolyte it is.
. H 2 O B. Which of these compounds is a strong electrolyte Select one. If the physical or chemical process that generates the ions is essentially 100 efficient all of the dissolved compound yields ions then the substance is known as a strong electrolyte.
Identify substance as acid base or both NH3. Complete the following reaction and identify the Bronsted acid. Which of these compounds is a strong electrolyte.
Which of the following compounds is a strong electrolyte. Which of these compounds is a strong electrolyte. CHCOOH acetic acid D.
A HCl D O 2 B CH 3COOH acetic acid E NaCl C C 6H 12O 6 glucose. Also It is strong base given in image attached. Molecular Compound Electrolyte Type Species in Solution sucrose table sugar non-electrolyte molecules only acetic acid HC 2 H 3 O 2 HOAc weak electrolyte molecules and some ions hydrogen chloride HCl strong electrolyte ions only 3.
Which of these compounds is a weak electrolyte. H 2 S O 4. Which of the following compounds is a weak electrolyte.
Principally they are in the form written when. C 6 H 12 O 6 glucose E. Potent potassium Potassium plays an important role in cell excitability regulation nerve impulse conduction resting membrane potential muscle contraction and myocardial membrane responsiveness intracellular osmolality control Fundamental phosphorus.
Which of these compounds is a strong electrolyte. I would not consider them as strong electrolytes. Also it is an ionic compound.
They include strong acids strong bases and salts. Which of these compounds is a strong electrolyte. HBr is strong acid Given in image attached.
Add your answer and earn points. CH 3 COOH acetic acid d. Hence it is not an weak electrolyte.
C H 3 C O O H acetic acid. B HBr. Hence it is not an weak electrolyte.
H 2 O b. But polar compounds are not always strong electrolytes. H2o o2 h2so4 c6h12o6 glucose ch3cooh acetic - Brainlyin.
H 2 SO 4 D. CH 3 COOH acetic acid. Strong electrolytes separate 100 into ions when they dissolve in water.
H2O N2 CH3COOH C2H6O KOH- RIGHT ANSWER. Answered Which of these compounds is a strong electrolyte. In fact these compounds are often only 15 dissociated.
C 6 H 12 O 6 glucose e. Which of these compounds is a strong electrolyte. Which of these compounds is a strong electrolyte.
Substances that do not yield ions when dissolved are called nonelectrolytes. Ionic compound are strong electrolytes if they are soluble in water. We can speak of strong and weak electrolytes depending on the degree of dissociation.
H2O O2 H2SO4 C6H12O6 CH3COOH. H2SO4 Sulphuric acid is a strong electrolyte as it is a strong acid. CH3COOH acetic acid 1 See answer Add answer 5 pts Advertisement S2s is waiting for your help.
Potassium phosphate and magnesium are among the most abundant electrolytes inside the cell. Strong acids and strong bases are strong electrolytes eg HClaq H 2 SO 4 aq HClO 4 aq. A H 2O D CH 3CH 2OH ethanol B N 2 E KOH C CH 3COOH acetic acid Ans.
C a F X 2 B a S O X 4 are ionic compounds but they are not soluble in water. Electrolyte strong electrolyte non electrolyte weak bases methylamine CH3NH2 aq electrolyte weak base. C 2 H 6 O ethanol e.
The compound potassium carbonate is a strong electrolyte. A strong electrolyte is a solutionsolute that completely or almost completely ionizes or dissociates in a solution. Tech and Engineering Blood tests.
CH3COOH acetic acid B. Answer 50 5 1 Brainly User Answer. KOH is again an ionic compound which has ions K and OH.
Which of these compounds is a strong electrolyte. СНCООН асetic acid С. These ions are good conductors of electric current in the solution.
These substances constitute an important class of compounds called electrolytes. It is sodium nitrate because it is a strong electrolyte that comes from a strong acid HNO3 and a strong base KOH. Strong acids strong bases and soluble ionic salts that are not weak acids or weak bases are strong electrolytes.
It becomes a strong electrolyte when it dissolves in water its formula is NaNO3 which is a compound that forms nitrate together with potassium nitrate and is obtained by chemical synthesis. Which of these compounds is a nonelectrolyte. Write the reaction when potassium carbonate is put into water Chemistry How do you assign oxidation states for each atom in the following compounds.

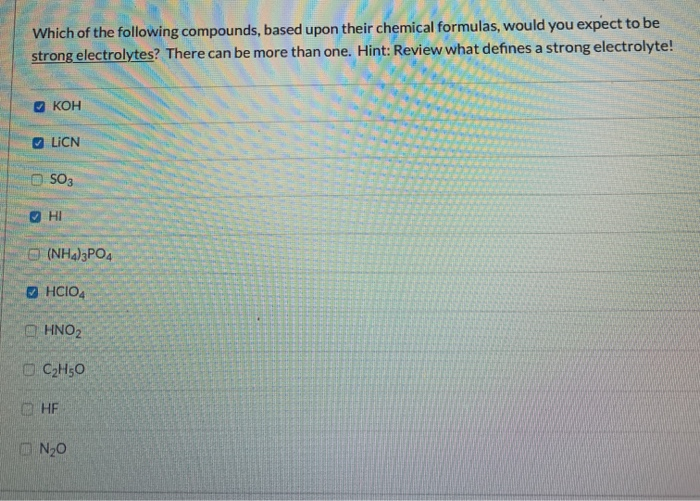
Solved Which Of The Following Compounds Based Upon Their Chegg Com


Belum ada Komentar untuk "Which of These Compounds Is a Strong Electrolyte"
Posting Komentar